Die jüngsten Durchbrüche in der Krebsforschung und -behandlung sind auf dem Gebiet der Immunonkologie zu verzeichnen, wo neuartige Immuntherapien eingesetzt werden, um das Immunsystem des Wirts zu stärken, damit es Tumorzellen wirksamer bekämpfen und zerstören kann. Zur Liste der von der FDA zugelassenen Therapeutika mit immunregulierender Wirkung gehören Antikörper- und Zytokin-basierte Immuntherapien, Kleinmolekül-Medikamente und zellbasierte Therapie. Der Erfolg, den diese und andere Therapien bei der Verlängerung der Überlebensdauer hatten, hat den Schwerpunkt auf die Entwicklung neuer Einzel- und Kombinationstherapien mit größerer Wirksamkeit bei der Behandlung von Krebs gelegt.
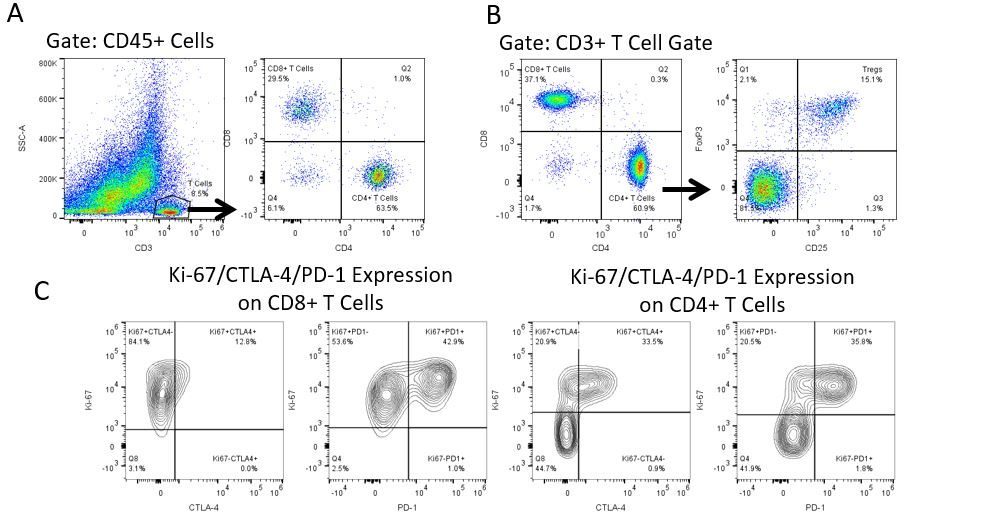
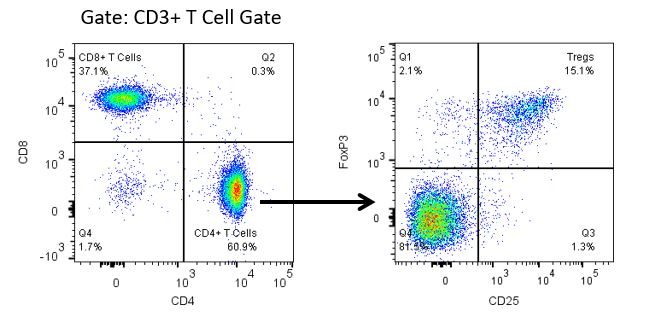
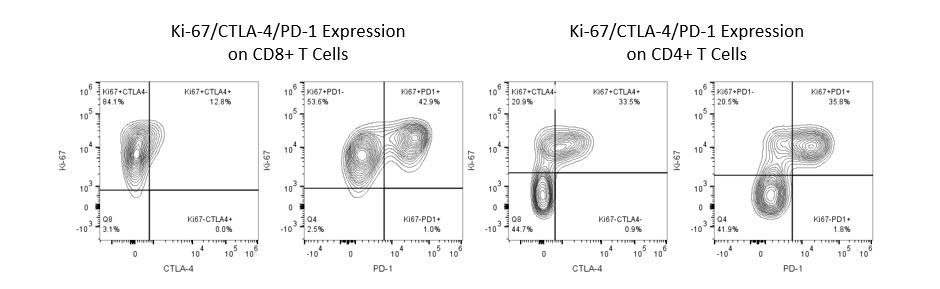
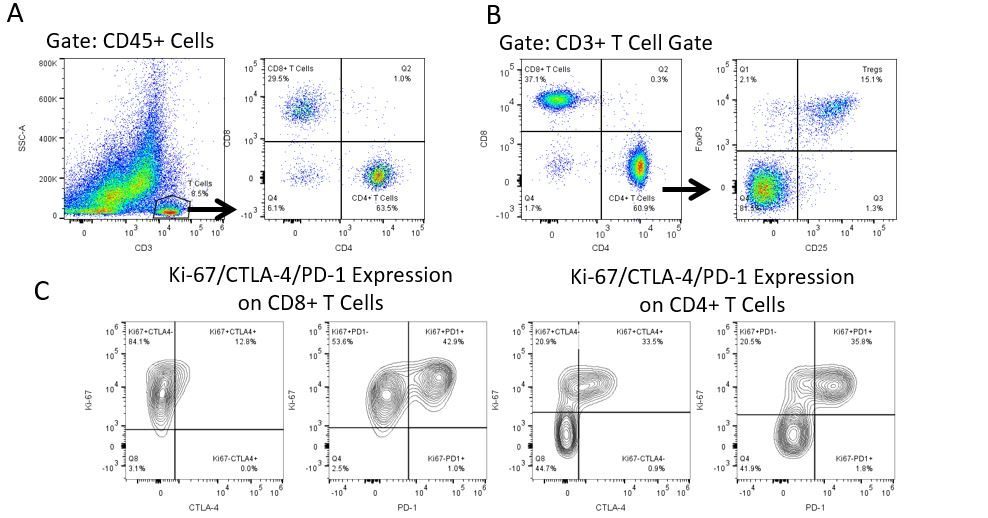
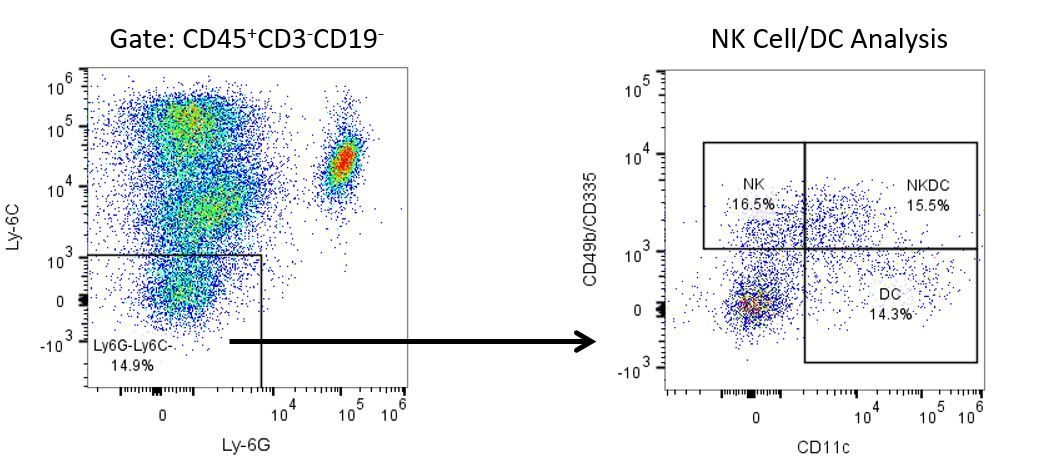
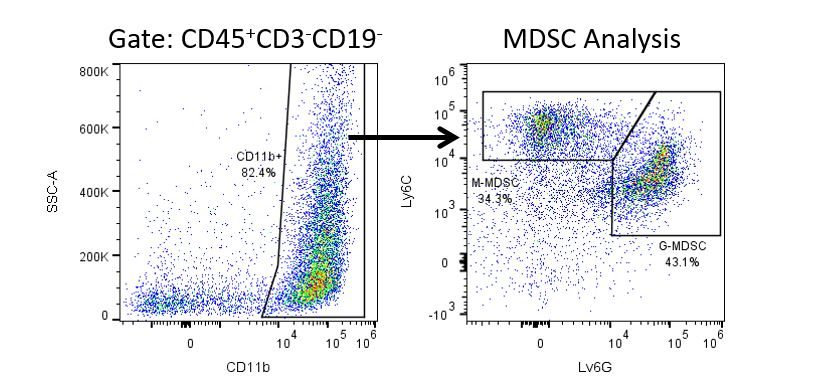
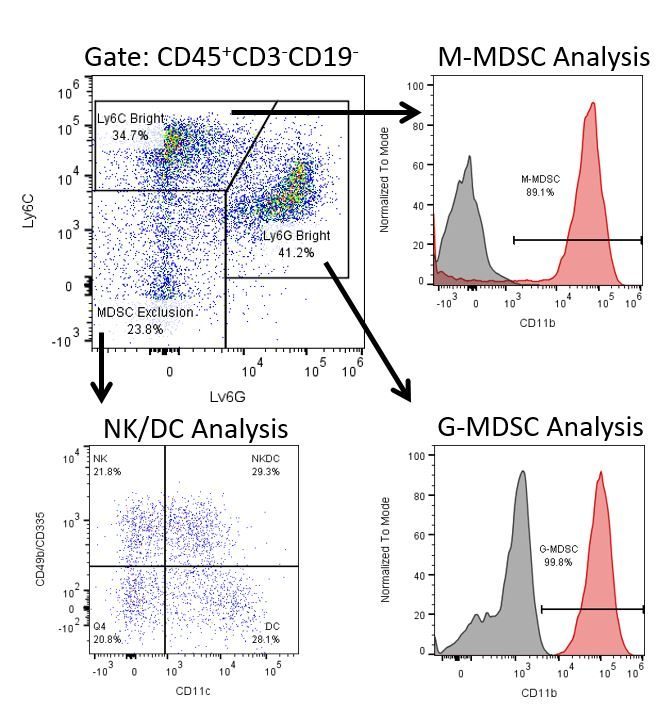
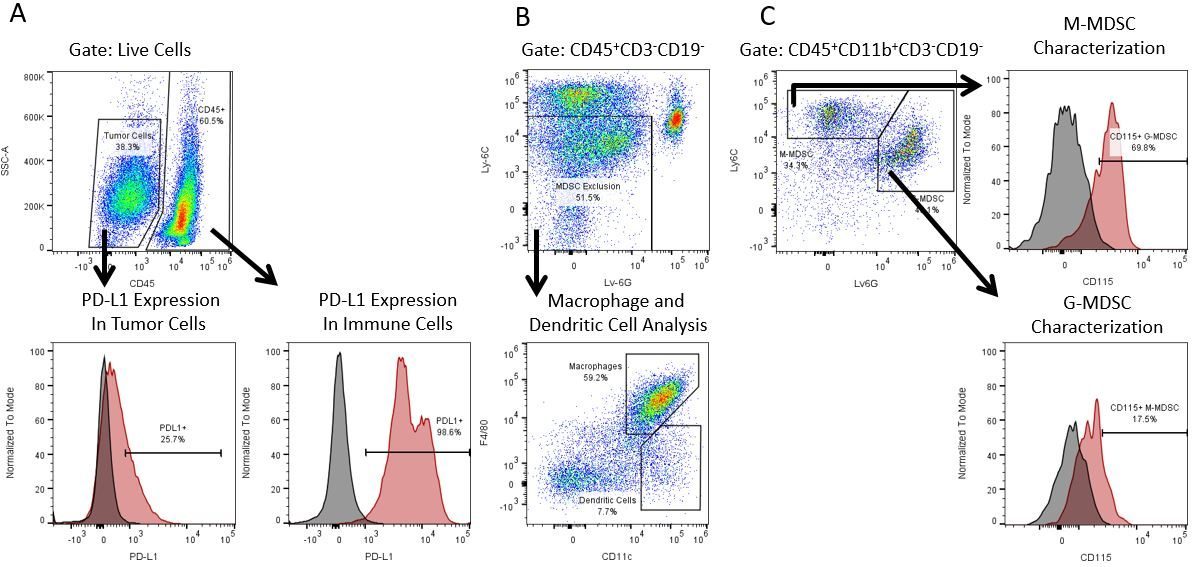
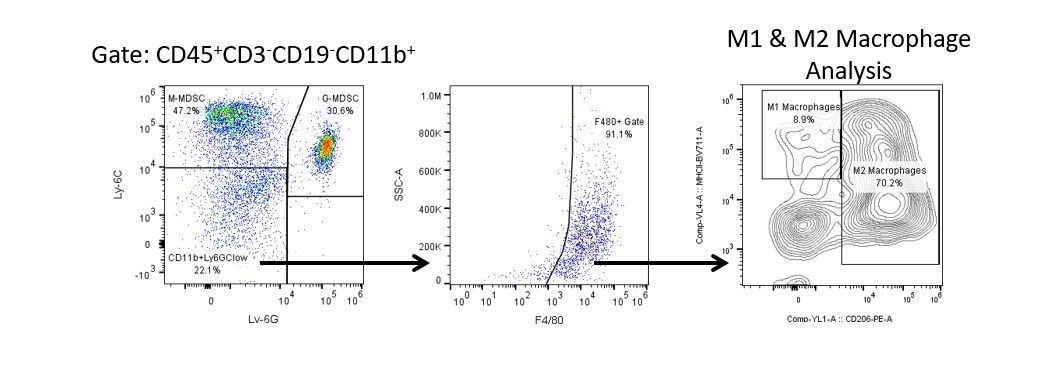
Die produktive Entwicklung von Immuntherapien erfordert einen hohen Durchsatz und eine robuste quantitative Analyse des Immunsystems in der Mikroumgebung des Tumors, dem peripheren Blut und anderen Wirtsgeweben.
Um diesen Bedarf zu decken, bietet Ihnen unser Vertrags-Durchflusszytometrie-Service eine fortschrittliche, hochmoderne analytische Durchflusszytometrie-Ressource zur Unterstützung aller Aspekte Ihrer Wirkstoffentwicklung. Führen Sie Ihre Studien zur Probengenerierung mit uns durch oder schicken Sie uns über Nacht Ihre vorklinischen oder nicht durch die CLIA regulierten klinischen Proben und wir übernehmen die Durchflusszytometrie für Sie.